Getter purification
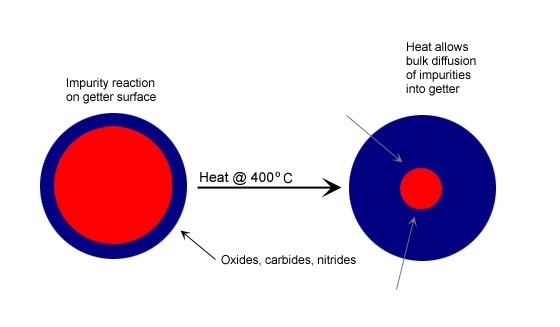
Zirconium alloys with aluminum (Zr-Al), iron (Zr-Fe), and vanadium (Zr-V-Fe) have been used for gas purification for over 30 years. The getters are typically used in a porous pellet form of various sizes. The zirconium metals are very reactive with a wide variety of gas species such as O2, H2O, CO, CO2 and N2. The chemisorption reactions are irreversible and form oxides, carbides and nitrides via the following reactions:CO + Zr -> Zr-O + Zr-C
CO2 + Zr -> Zr-C + 2 Zr-O
N2 + Zr -> 2 Zr-N
O2 + Zr -> 2 Zr-O
Hydrogen sorption is governed by a different reaction where hydrogen is dissociatively chemisorbed and dissolved into the bulk of the metal getter:
H2O + Zr -> Zr-O + 2H (bulk)
Zirconium getters are operated at elevated temperatures, typically 350-450C, depending on the gas to be purified and impurities to be removed. The chemical reactions shown above occur on the surface of the metal, and the reaction products then diffuse into the bulk structure, providing clean surface for renewed adsorption and reaction. This reactivation process is carried out continuously at high temperatures.
The capacity of getter can be as much as 25 times that of ambient metal-supported catalyst media since getter can use the bulk of the media for adsorption while other catalysts rely only on surface reations.
Outlet purity from a getter purifier has been shown to be in the single digit ppt range under optimum operating conditions. Because of its' ability to removal virtually all impurities from rare gases, getter media is used to provide zero gas for the most sensitive analytical instrumentation.
Getter purifiers are used for rare gases including argon, helium, krypton, xenon as well as nitrogen and hydrogen. The most common application for heated getter purifiers is rare gases since it can remove nitrogen, hydrogen and hydrocarbons.
| Advantage | Benefit |
| Longer lifetime than ambient catalyst media helps reduce maintenance frequency | Provides lower cost-of-ownership and allows use of smaller vessels to reduce cost and overall footprint |
| Only technology that can remove N2 and all hydrocarbons from rare gases such as argon and helium | Prevents nitride and carbide inclusions during crystal growth and sputtering processes |
| Heating and control system used to maintain getter temperature also provides continuous monitoring and feedback on purifier performance | Easy to confirm purifier performance |
| Limitation | Response |
| Outlet purity can decrease momentarily during spikes in flow or inlet impurity due to reduced residence time of the gas within the getter vessel. | Be sure the purifier is properly sized so that maximum expected flows are still well within the maximum flow rating of the purifier |
| Efficient removal of impurities such as CH4, CO and CO2 may be compromised at higher inlet levels. | Be sure the getter vessel is sized to provide adequate residence time for removal of all impurities at maximum expected inlet levels |
| Heating and control system increase initial purchase price and utility requirements as compared to ambient catalyst media | Costs recovered over time due to longer lifetime, reduced downtime and elimination of regeneration steps |
| Application | Gases Used | Process Benefit |
| Silicon crystal growth | Argon | Removes oxygen, carbon and nitrogen impurities to prevent crystal defects |
| Silicon carbide crystal growth | Argon | Removes oxygen, carbon and nitrogen impurities to prevent crystal defects |
| Physical Vapor Deposition and Sputtering | Argon, Nitrogen | Argon purifiers remove oxygen, carbon and nitrogen impurities to improve wafer uniformity. Hydrogen removal helps improve process chamber pumping speeds. Nitrogen purifier helps prevent introduction of impurities during loadlock purge sequence. |
| Zero gas for analytical instruments | Nitrogen, Argon | Removes all impurities to provide baseline for APIMS, mass spectrometers and other ppb-level instruments |
| Optical Fiber Manufacturing | Helium | Removes all impurities to prevent loss of attenuation in optical fiber |




 BACK TO PURIFICATION TECHNOLOGIES
BACK TO PURIFICATION TECHNOLOGIES